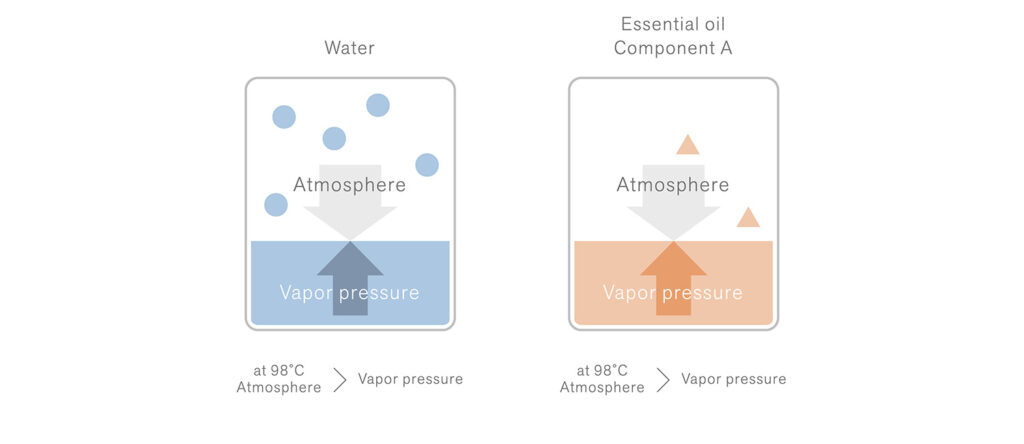
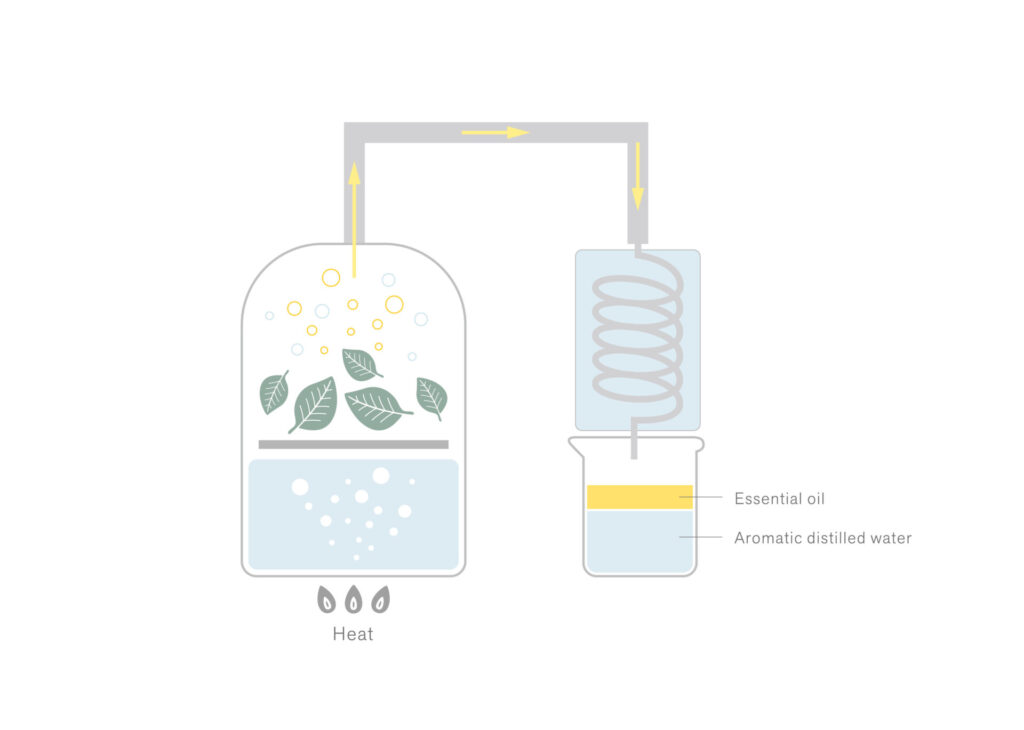
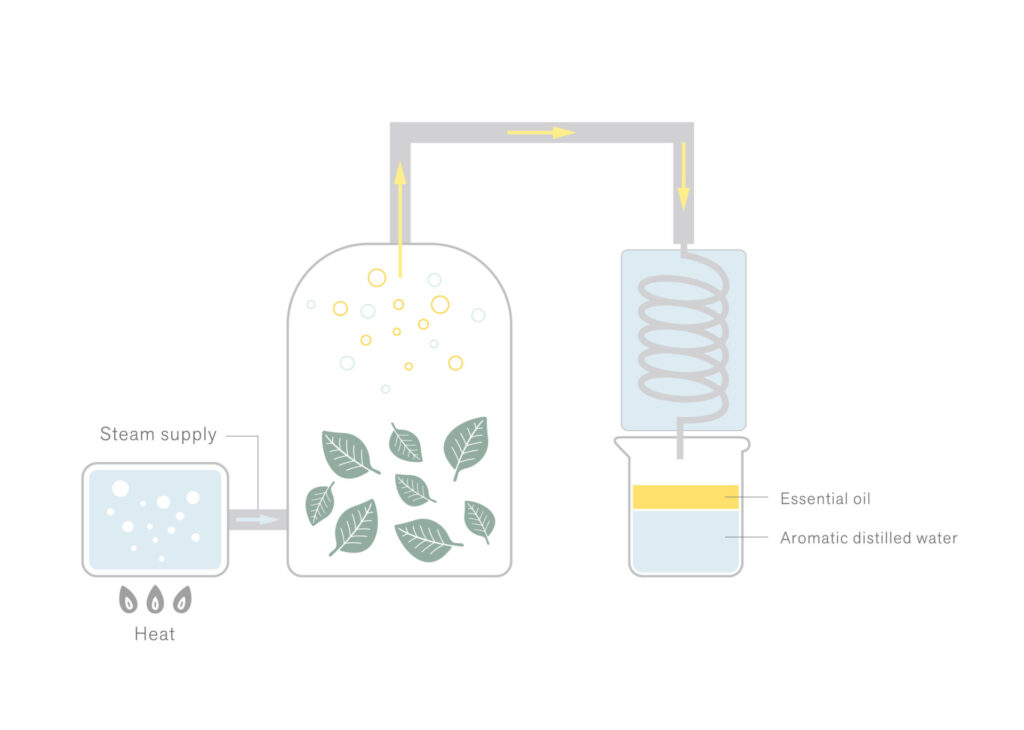
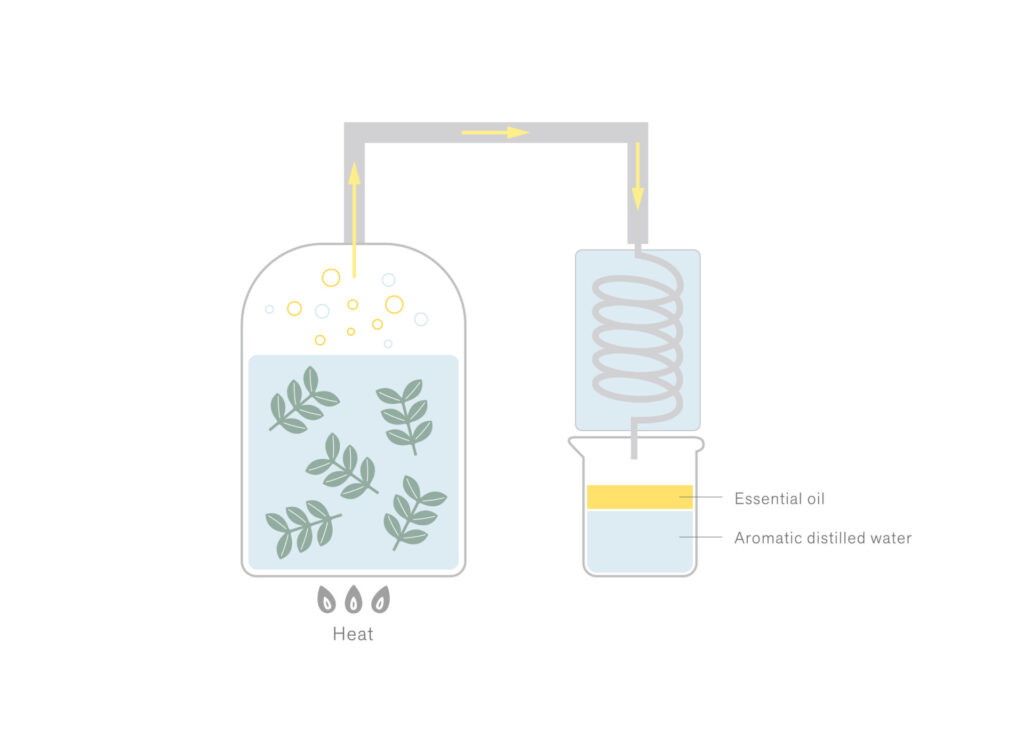
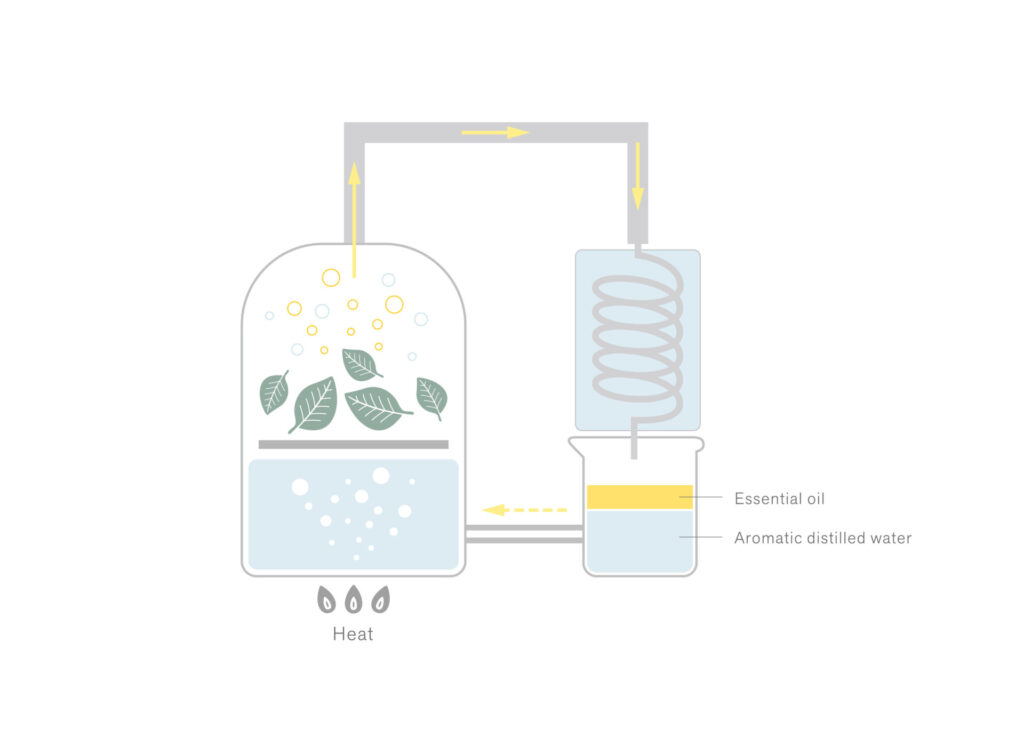
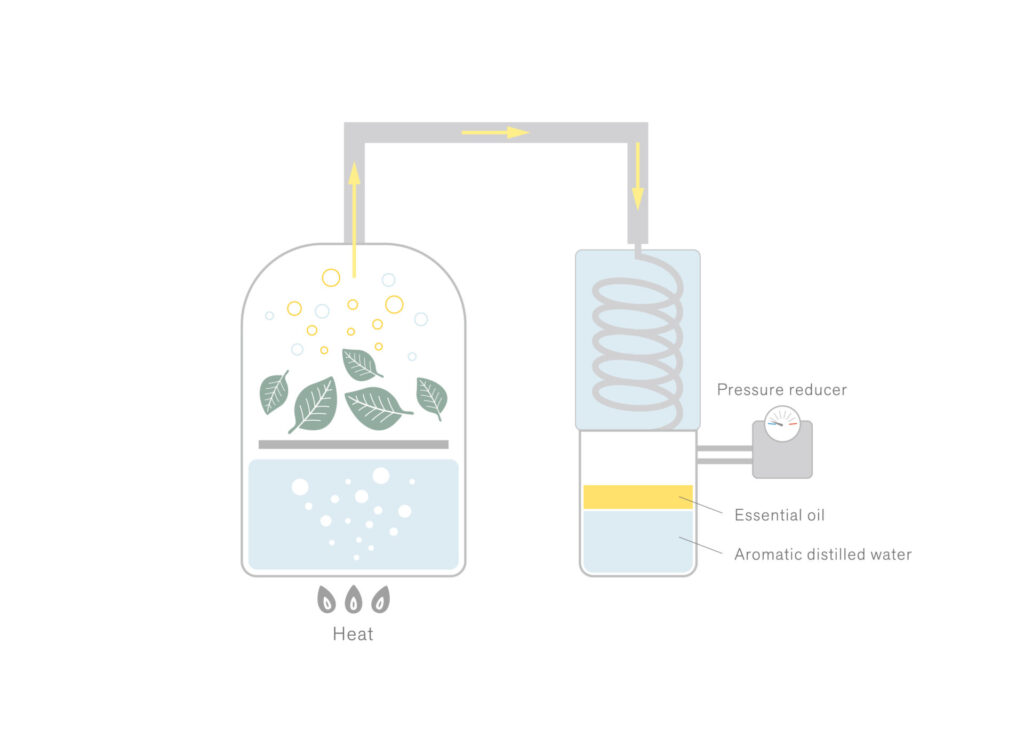
The boiling point of aromatic components is typically higher than that of water. However, when they are heated alongside water, they can transition into a gaseous state at a lower temperature than their individual boiling points (approximately equivalent to the boiling point of water).
Example: Steam distillation of essential oil component A (boiling point at 200℃)
At 98°C, both water and essential oil component A exhibit vapor pressures that are slightly lower than atmospheric pressure, indicating that neither of them has yet reached its respective boiling point.