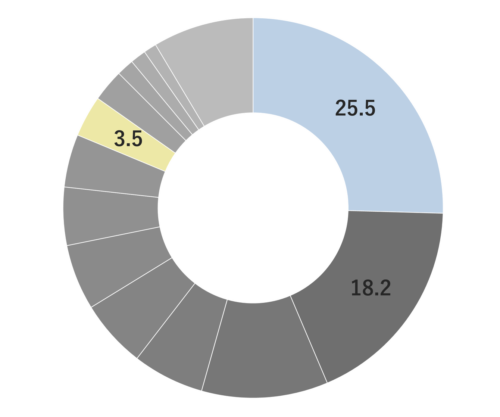
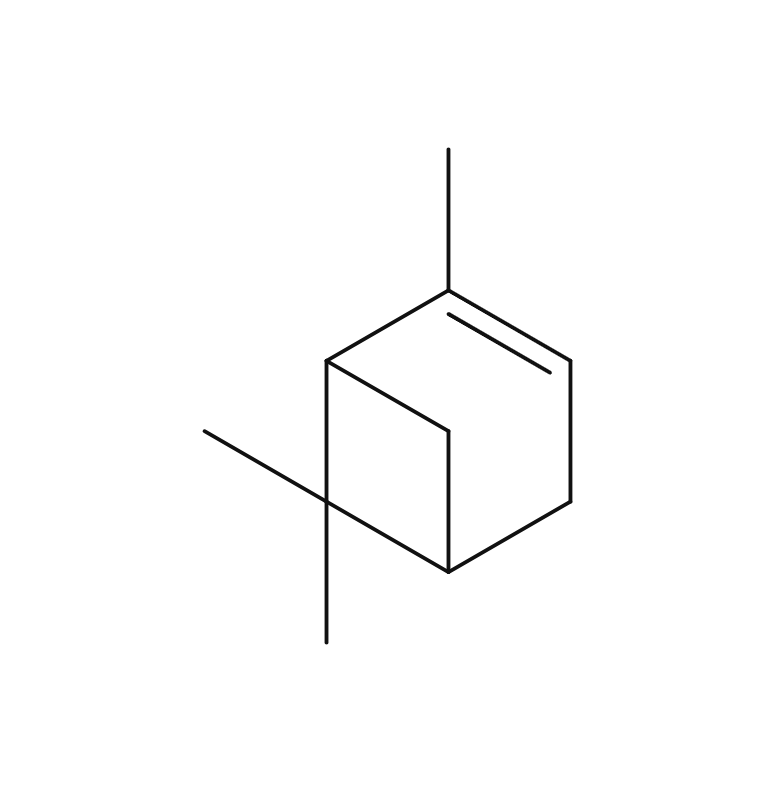
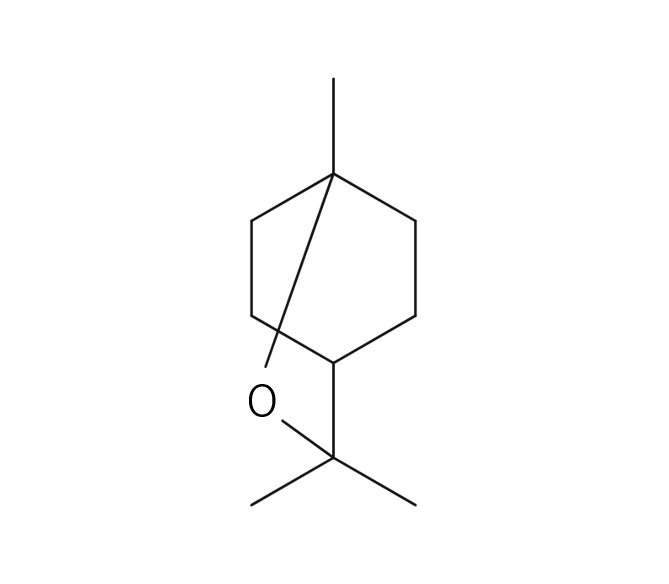
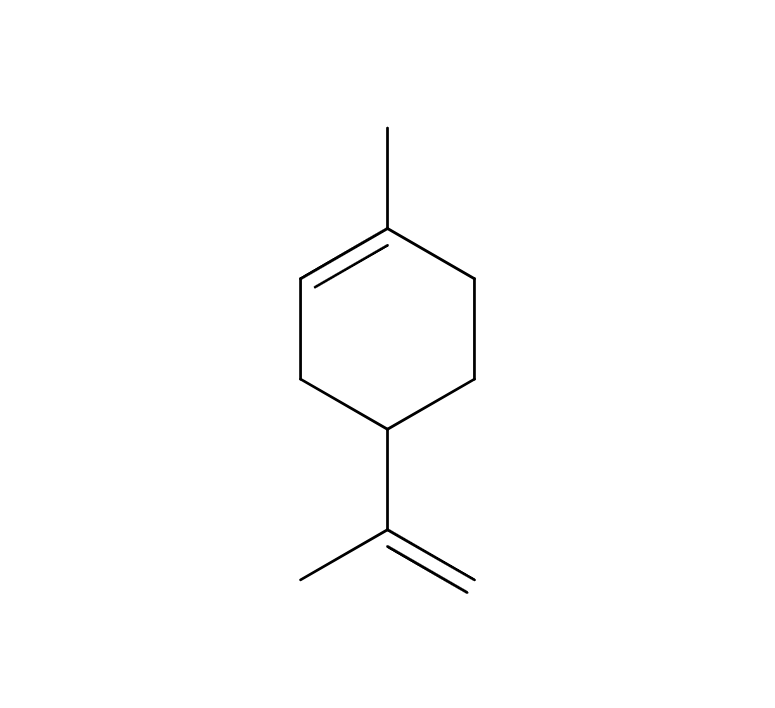
Sage is a perennial evergreen herb belonging to the Lamiaceae family, reaching up to 60 cm in height. It boasts distinctive grayish-green, oval leaves and vibrant purple flowers. The plant’s scientific name, “Salvia,” originates from the Latin word “Salvus,” signifying “safe and healthy.” Sage has a rich historical significance as a medicinal herb, finding extensive use in ancient Arabia and ancient Greece. Additionally, it was employed as a protective amulet. Sage exists in numerous varieties, such as “Spanish Sage” and other horticultural species. Among these, “Dalmatian Sage” is considered the most aromatic among the different varieties.