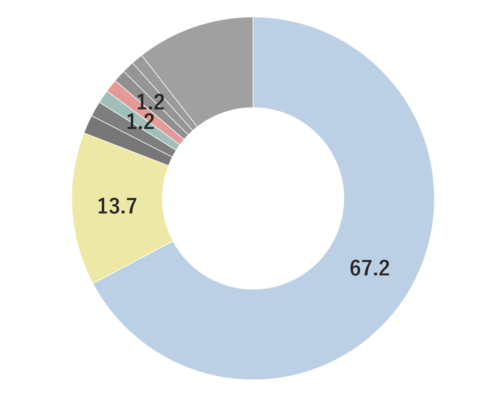
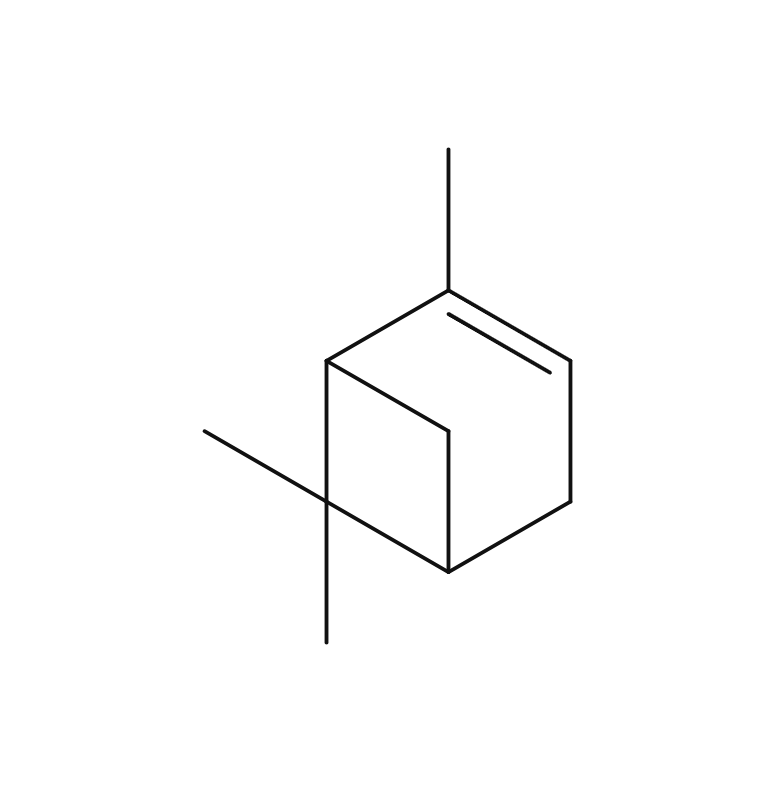
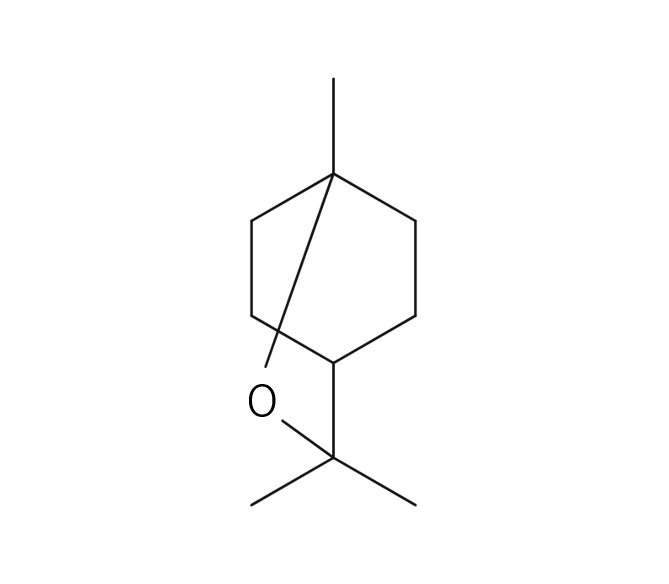
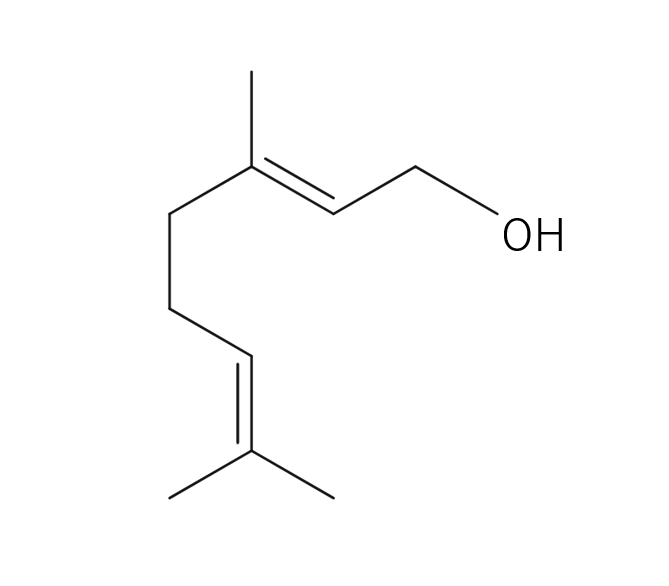
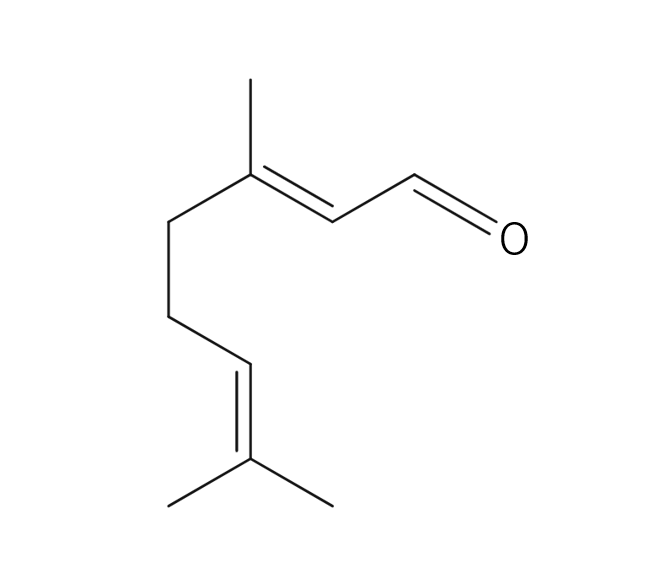
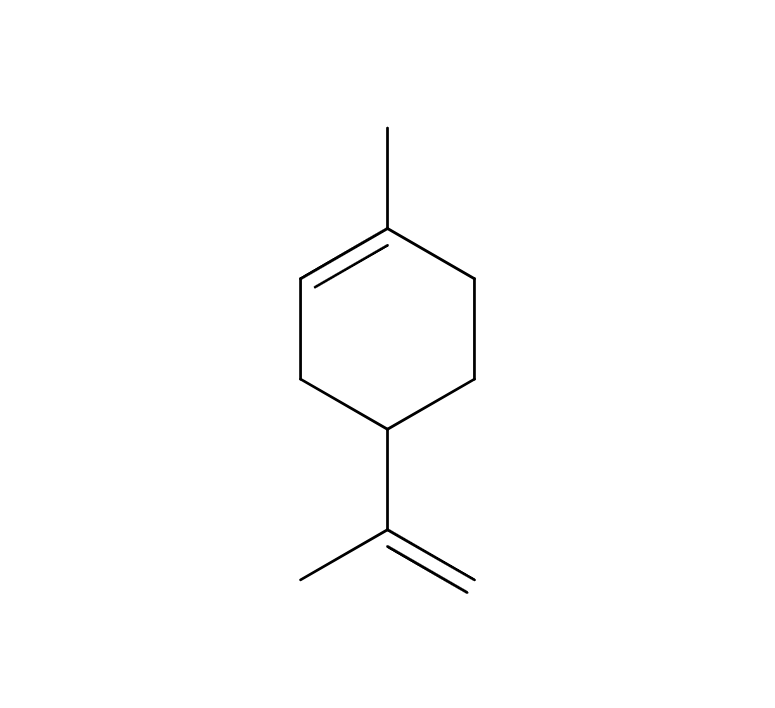
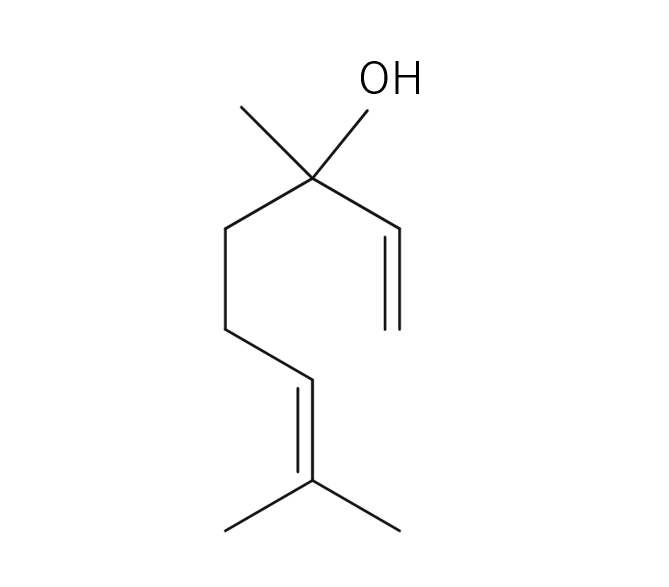
About 3-5% of May Chang's essential oil is obtained through steam distillation from May Chang fruit, resulting in a pale yellowish hue. Its primary constituents include geraniol, neral, limonene, and linalool. The fragrance is well-balanced, featuring a dark lemon-like green depth along with a hint of vanilla-like sweetness. It harmonizes effectively with various essential oils, but it particularly complements woody scents such as Patchouli and Sandalwood, known for their sweetness and depth. It also pairs beautifully with other deep, sweet fragrances like Ylang-Ylang and Geranium. Due to its potent fragrance, it is advisable to add it gradually when blending to achieve a balanced scent.