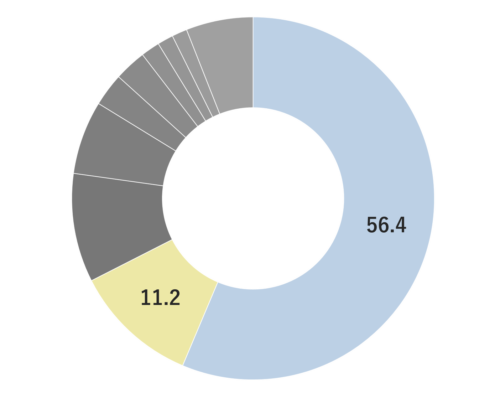
Stress Reduction
A study reported that mice subjected to cold stress at 4°C (41°F) exhibited reduced elevations in blood corticosterone levels when treated with limonene. Furthermore, the same report demonstrated that limonene effectively lowered corticosterone levels in the blood of mice exposed to both physical and mental stress.1)
Memory Improvement
A study reported that when acetylcholinesterase reacts with acetylthiocholine in phosphate-buffered saline, the introduction of limonene inhibits the degradation by acetylcholinesterase. Acetylcholinesterase plays a role in the breakdown of acetylcholine, a hormone associated with memory and learning. In the same report, it was also noted that limonene suppressed memory impairment induced by scopolamine administration in rats.2)
Anti-cancer Effects
A report indicated that the consumption of limonene by breast cancer patients resulted in reduced expression of Cyclin D1, a protein crucial for cell division, within tumors.3)Furthermore, numerous other studies have also documented the anticancer properties of limonene.4)5)