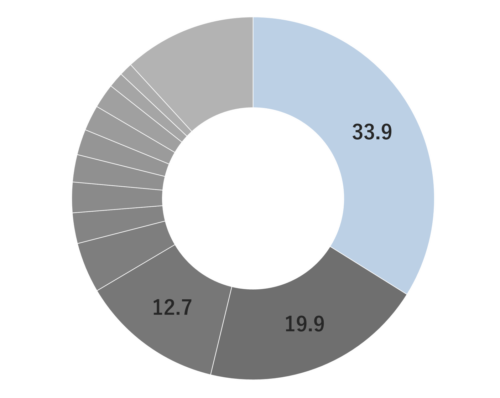
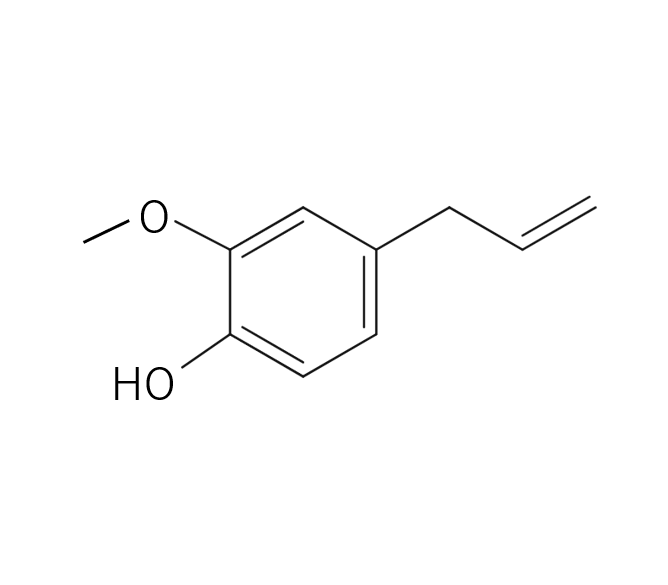
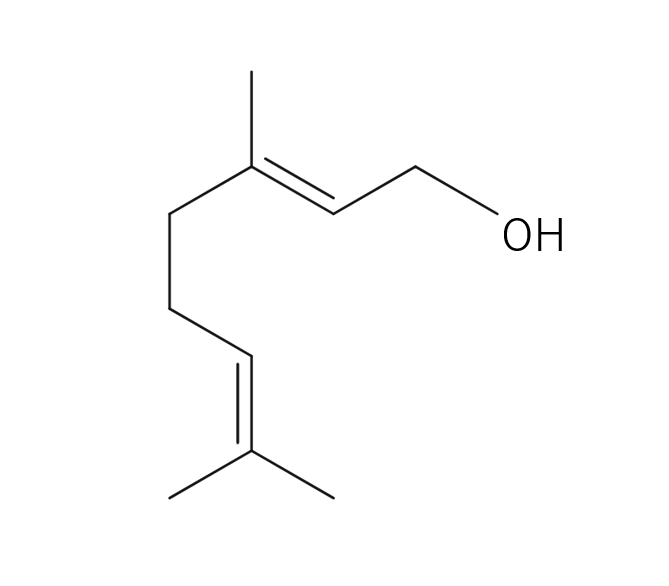
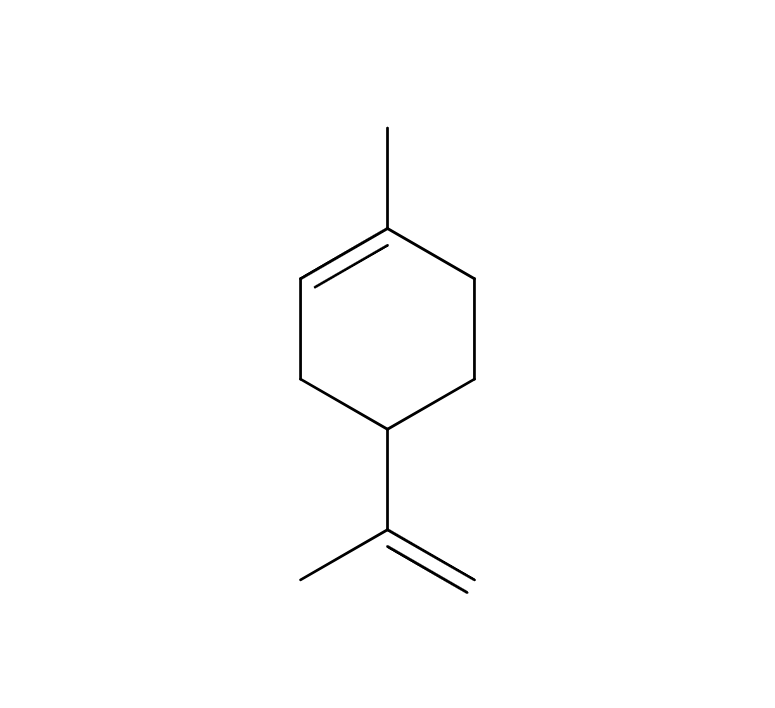
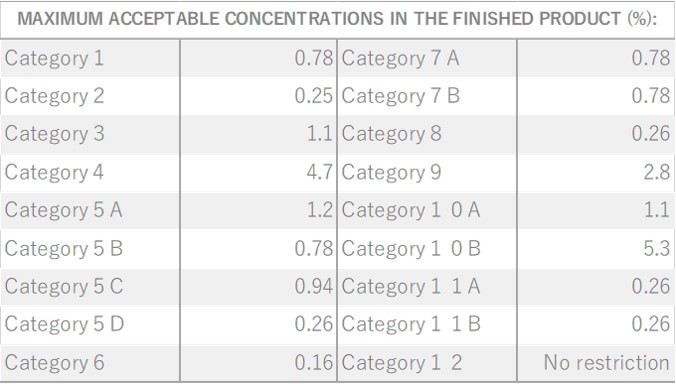
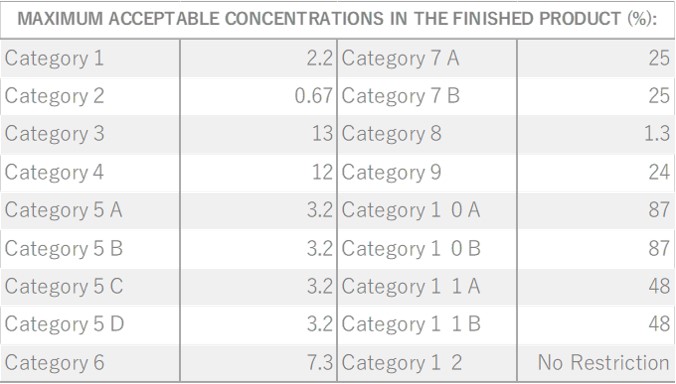
Citronella, belonging to the Poaceae family, reaches a height of approximately 1 meter. Its leaves are characterized by their elongated, green appearance, a common trait within this plant family. The scientific name “Citronella” is believed to have been inspired by its fragrance, reminiscent of the citrus fruit “Citron.” The genus name “Cymbopogon” is derived from two Greek words, “Kymbe,” meaning ship, and “pogon,” meaning beard, alluding to the ship-shaped and bearded nature of the plant. It is renowned as one of the most widely cultivated fragrant plants globally. Close botanical relatives of citronella include Lemongrass and Palmarosa. Citronella comes in two main types: Ceylon and Java, with some asserting that the Java variety offers superior yields and oil content. In the Western regions, it is well-known and utilized as an effective insect repellent.