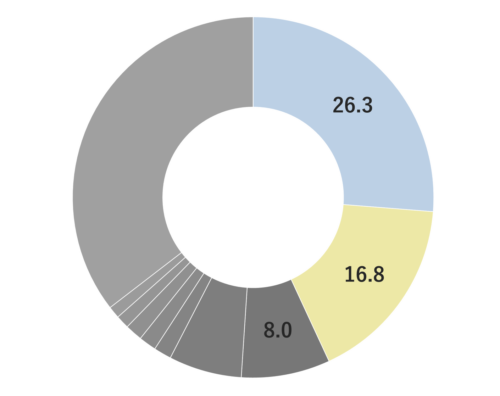
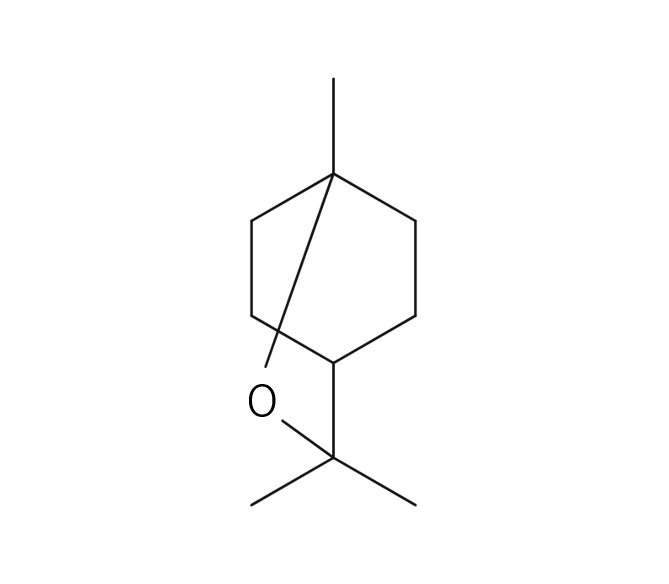
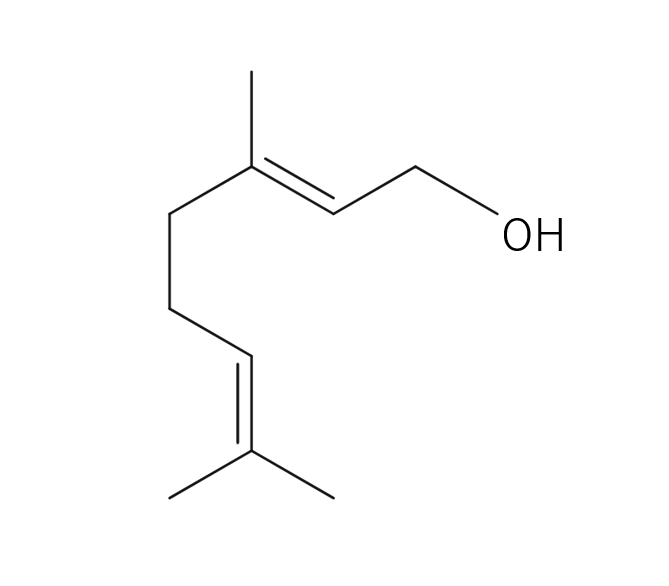
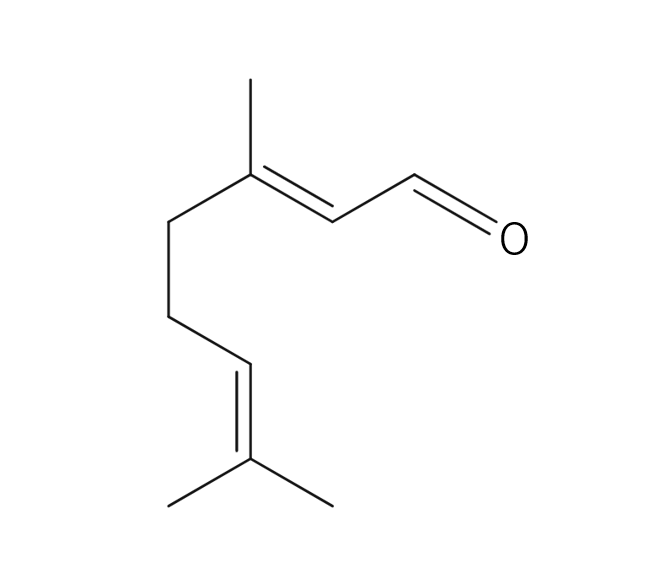
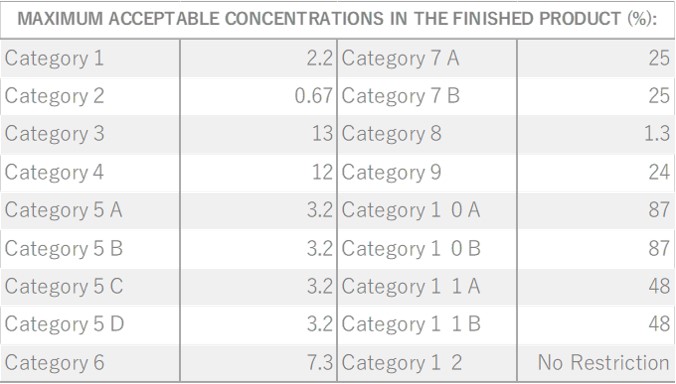
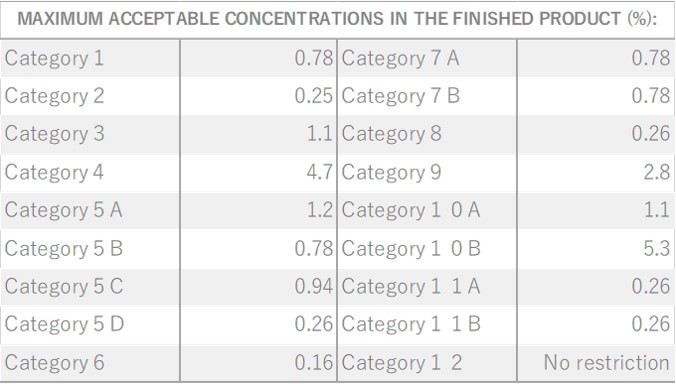
Damask Rose, belonging to the Rosaceae family, is a shrub reaching a height of 2 meters. It features a diverse array of flower varieties, each displaying unique colors and aromatic intensities. Among these, the most notable is the “Damask rose,” due to its incredibly potent aroma. These palm-sized flowers are pink and exude a sweet, rich, and fragrant scent. The scientific name “damascena” is believed to originate from “Damas,” a reference made when the rose was introduced to Bulgaria. It was subsequently named “Rosa damascena” in Latin. Roses have been intertwined with human history since 3000 B.C., appearing in numerous myths and deeply influencing people’s lives through various uses, including rituals and medicinal purposes.