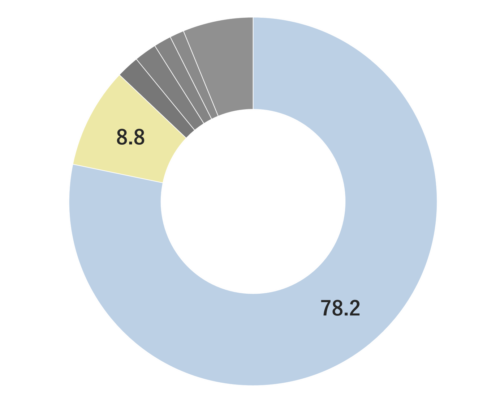
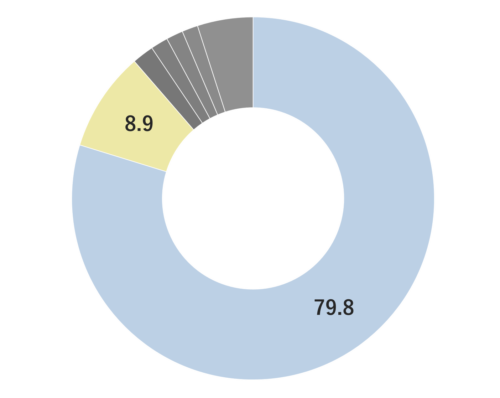
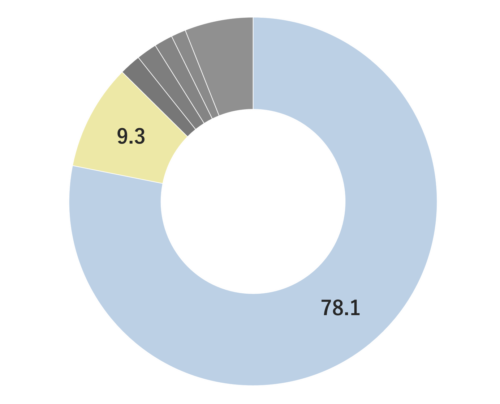
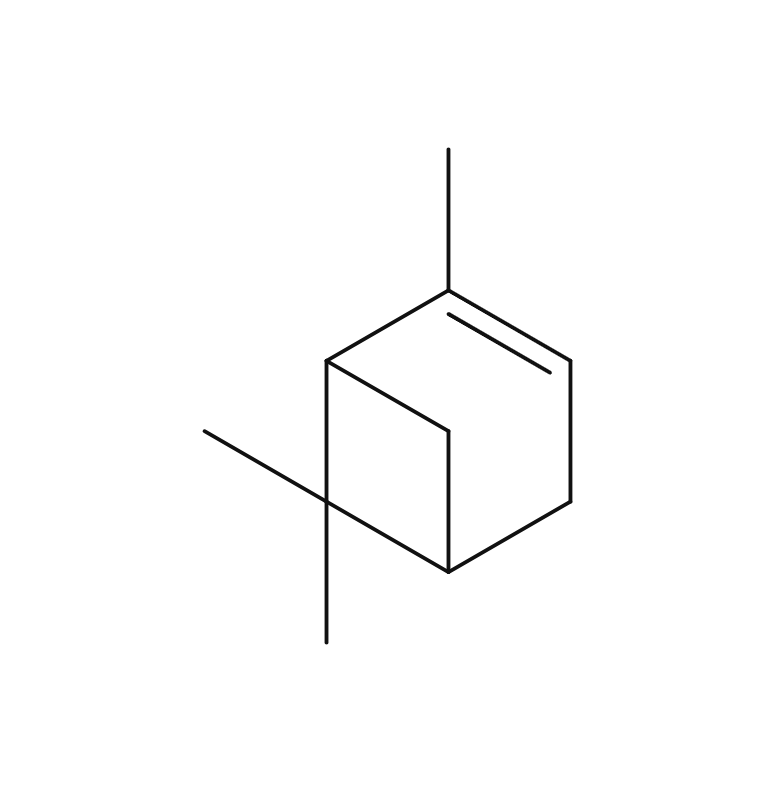
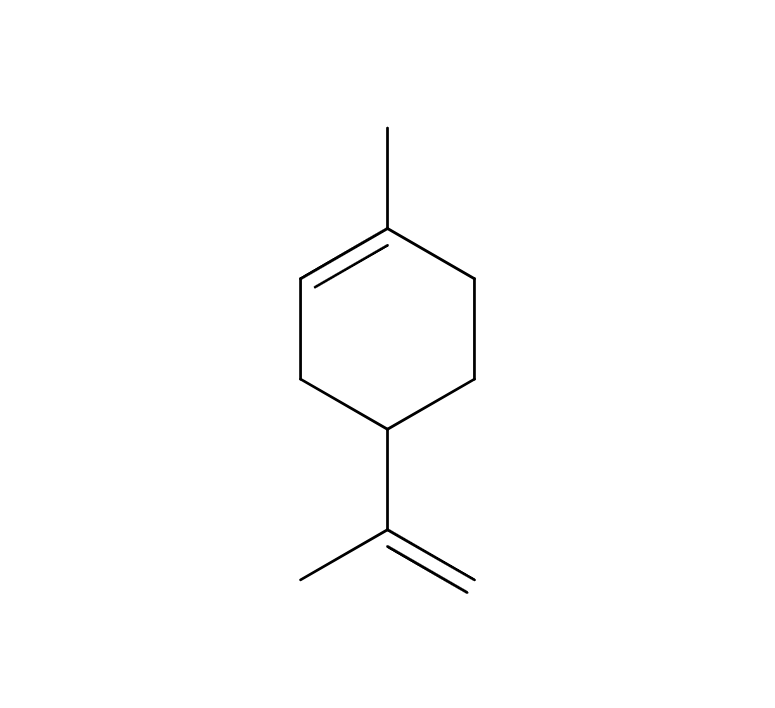
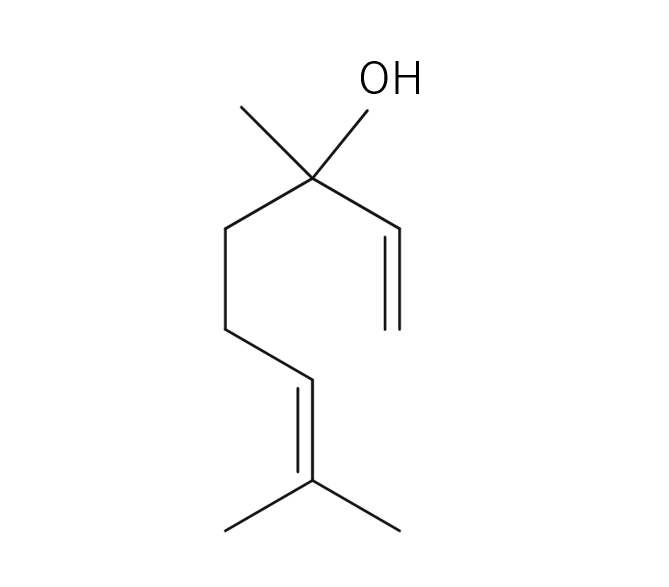
Yuzu essential oil, obtained from its pericarp through steam distillation, comprises approximately 0.2% of the total yield and carries a yellowish hue Its primary components are limonene and gamma-terpinene. Limonene is commonly used in products like detergents. The aroma is truly unique, offering a fruity tone with freshness and a subtle bitterness that imparts a warm, relaxing sensation. Through steam distillation, the essential oil acquires a slightly lighter, fresh, and clear character. This versatile oil seamlessly blends with various scents, including woody fragrances like Hinoki, as well as distinctive aromas such as Juniper and Rosemary. Due to its relatively mild fragrance, it is advisable to add a bit more of this essential oil to maintain a well-balanced aroma blend. It is used mainly in skin care and various other applications.