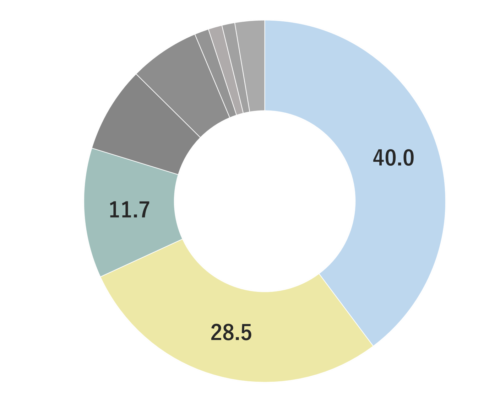
Bergamot belongs to the Rutaceae family and their trees grow up to around 4 meters tall. Alongside its distinctive yellow-green fruit, it has green leaves with small white flowers. The fruit is renowned for its extreme sourness, rendering it inedible in its natural state. Due to its delicate and challenging cultivation, Bergamot is often primarily used for fragrances. The origin of the name “Bergamot” can be traced back to Bergamo, Italy, where the tree was first cultivated. It is predominantly grown in Calabria, Italy, along the coasts of the Ionian Sea and the Thionian Sea. Other regions include Brazil, Cote d’Ivoire, Morocco, Portugal, and Guinea.