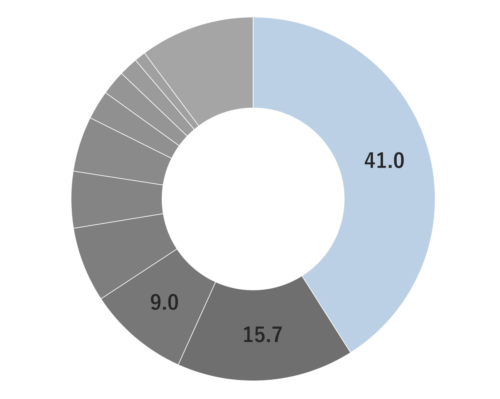
Laurel belongs to the Lauraceae family and grows up to about 10 meters tall. It produces oval green leaves all year round. The flowers are small and yellow and bloom in clusters. The fruits are small and black when mature. Laurel holds significant cultural and historical importance, having been regarded as the sacred tree of the god Apollo in ancient times. This association led to the tradition of crowning winners of the ancient Greek Olympic games with laurel branches and leaves. Laurel leaves, also known as bay leaves in culinary contexts, are commonly used as a flavorful spice ingredient. The name “laurel” is believed to have its origins in the Celtic word “laur,” which translates to “green.” While its origins can be traced to the Canary Islands in the Mediterranean Sea, it spread as an herb and spice and is now primarily cultivated in regions along the Mediterranean coast, including Turkey, Greece, Italy, France, and other coastal areas.