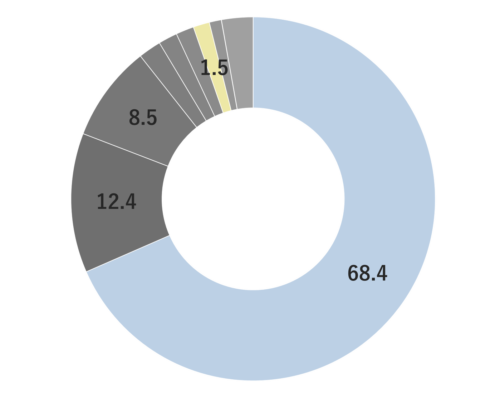
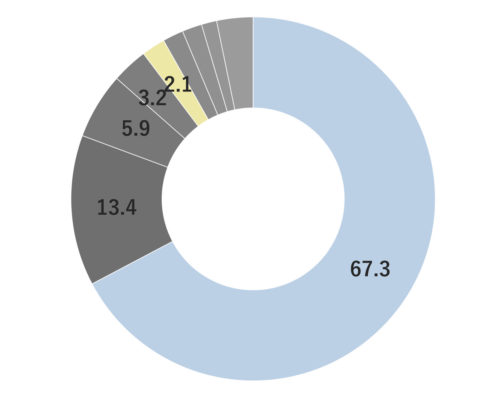
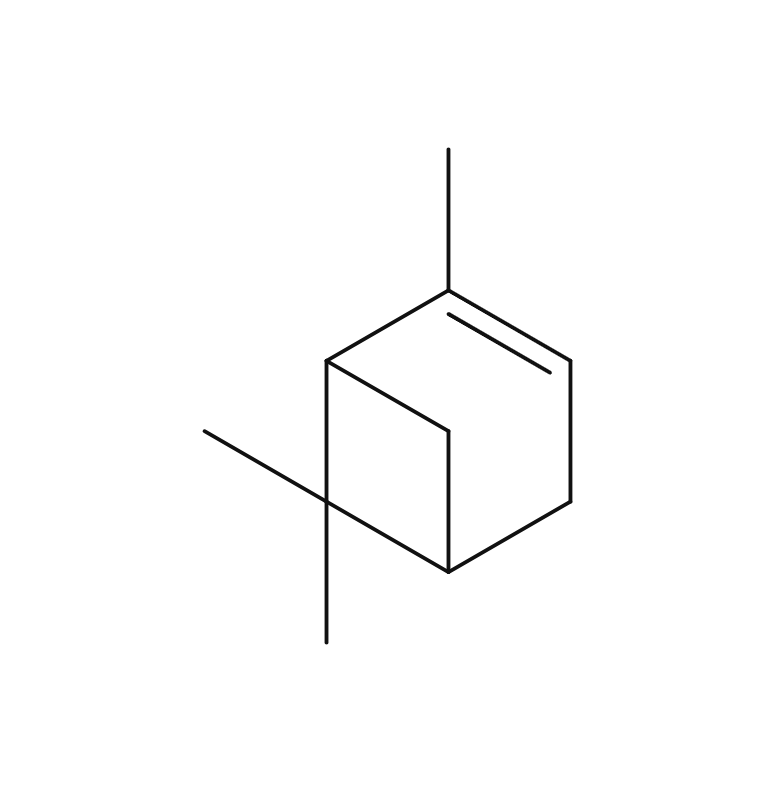
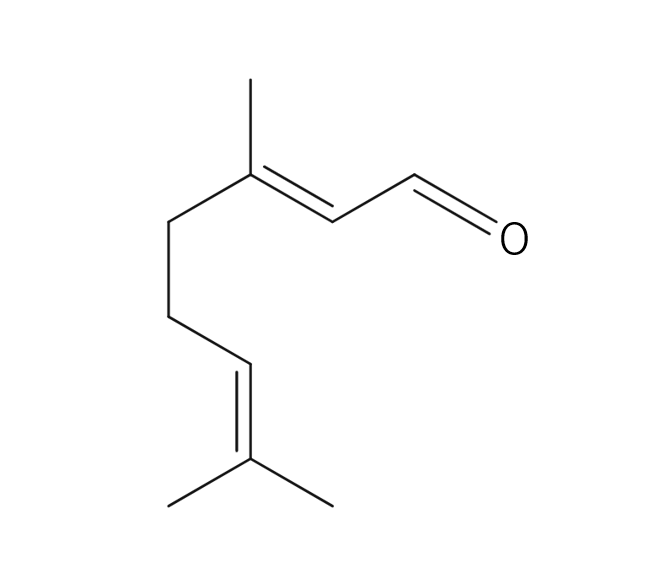
About 0.3 - 0.4% of Lemon essential oil is obtained through Cold Press from its peel and is colorless to pale yellow. Its primary components, limonene and citral, impart a delightful citrus aroma. It has a clear, clean, and invigorating aroma, with a refreshing lemon-like acidity and a hint of bitterness. When furocoumarins are removed, resulting in a phototoxic ity-free fragrance, the scent becomes slightly lighter compared to regular pressed oils. Therefore, for aromatic purposes, Cold Press is recommended. It blends harmoniously with various other essential oils, but its fragrance might appear subtler than others. When blending, careful consideration of the strength and quantity is necessary to achieve a balanced aroma. Given its relatively mild scent, adding a bit more to the blend can help maintain a well-balanced scent. This versatile oil is used in food flavors, cosmetic fragrances, diffusers, and detergents.